Leveraging the body’s natural healing mechanisms to treat diseases of the eye
Welcome to
OCUGENIX
1
Wet Macular Degeneration
2
Geographic
Atrophy
3
Proliferative Diabetic Retinopathy
The company has initiated a Phase II program for wet macular degeneration and anticipates reporting preliminary results by the end of 2025.
Ocugenix has an additional ocular indication in Dry Eye, which is in the IND-enabling stage.
How We Are
DIFFERENT
Ocugenix is the only company in ophthalmology that seeks to harness the body’s natural wound-healing processes to restore homeostatic functioning in the eye.
Our agonists activate signaling pathways that counter chronic inflammation and promote regenerative repair. Other drugs in these target indications inhibit specific symptoms of their disease, such as the edema that is the result of leakage in the pathological vessels. By targeting a single symptom or disease process, such as VEGF or complement, approaches are limited because these diseases are multifactorial. Our therapies are disease-modifying by pharmacologically coopting the entire physiologic cascade that restores homeostasis.
Wet AMD
Our initial target indication, wet AMD, reflects this difference in approach. Current anti-VEGF therapies counter the excessive amounts of VEGF, causing the aberrant blood vessels to constrict, thus reducing the edema, the cause of the initial symptoms of wet AMD. Unfortunately, these anti-VEGF agents do nothing to eliminate these pathological blood vessels and do nothing to reduce the development of fibrosis, which is the cause of long-term blindness that all wet AMD patients will ultimately develop. Only OGX-110 activates the processes that shut down and remove the pathological vessels and fibrosis formation.
Geographic Atrophy
- In Geographic Atrophy, complement cascade inhibitors slow the rate of growth of the geographic lesion by about 25%. Unfortunately, many other factors outside of the complement system contribute to this lesion growth, and these factors remain active even when the complement system is blocked.
- Only OGX-110 shuts down the chronic wound response to initiate resolution, blunting the effect of all inflammatory factors and other stimulants of lesion growth.
Dry Eye
- In Dry Eye, inflammation on the surface of the eye (caused by a variety of factors) results in significant discomfort to the patient.
- Only OGX-110 has shown the ability to end the inflammation and support the regeneration of the stem cells. It has the unique ability to shut down the disease at its source and restore a healthy micro-environment.
- This activity has been demonstrated in extensive animal studies.
- Only OGX-110 activates the processes that shut down and remove the pathological vessels and fibrosis formation.
- In Geographic Atrophy, complement cascade inhibitors slow the growth of the geographic lesion by about 25%. Unfortunately, many other factors outside of the complement system contribute to this lesion growth, and these factors remain in effect even when the complement system is blocked.
- Only OGX-110 shuts down the chronic wound to initiate resolution, blunting the effect of all inflammatory factors and other stimulants of lesion growth.
- In Dry Eye, inflammation on the surface of the eye (caused by various factors) results in significant discomfort to the patient.
- Only OGX-110 has shown the ability to end the inflammation and support the regeneration of the stem cells. It has the unique ability to shut down the disease at its source and restore a healthy micro-environment.
Science
OGX-110 is a small-molecule drug candidate that selectively activates the CXCR3 pathway, the pathway most strongly implicated in pruning neovascularization, limiting fibrosis, and restoring homeostasis in wound healing.
While existing treatments for these three targeted diseases block a single growth factor that drives vascular proliferation (generally VEGF), OGX-110 targets a pathway that overrides the numerous factors that drive angiogenesis and fibrosis. By activating CXCR3, OGX-110 induces the regression of abnormal blood vessels and fibrotic tissue, preserving the pre-existing vascular and fibrotic infrastructure and thereby restoring homeostasis.
The Wound Healing Paradigm
Like every biological process in the human body, wound healing occurs through specific and highly coordinated phases: hemostasis/inflammation, tissue replacement, and resolution. For a wound to heal properly, all phases must happen in the correct order and within the right time frame. During this final phase, wound resolution, all the cell and matrix resources recruited during tissue replacement diminish, returning the system to homeostasis. In this phase, over 90% of the vasculature and two-thirds of the matrix regress as the tissue regenerates.
Specific signals initiate this resolution phase by activating a cell surface receptor, CXCR3, which is the most important. Activation of this pathway is triggered by naturally occurring chemokines, with IP-10 being the main one. This signaling axis, IP-10, and CXCR3 are acute-phase molecules that are significantly upregulated to end the tissue replacement phase of wound healing and begin resolution. Failure to properly activate this axis leads to chronic inflammation, fibrosis, and permanent scarring. This occurs when the wound bed fails to sense completion through contact inhibition and remains in the tissue replacement phase. By pharmacologically activating the CXCR3 signaling pathway, it is possible to restore chronic wound processes to a state of homeostasis.
Our Target Indications
Wet Macular Degeneration
About 200,000 new cases of wet AMD are diagnosed each year in North America. Due to the aging population, the National Eye Institute estimates that the prevalence of advanced AMD will grow to 5 million by 2040.
In wet AMD, an aberrant wound response leads to the proliferation of blood vessels and a fibrotic growth starting in the macula, the area of the retina responsible for central vision. Damage to photoreceptors in the macula can cause distortion or blind spots in a person’s central vision. Patients with wet AMD who are untreated or for whom treatment is ineffective (about 30% to 50%) can progress to total blindness. VEGF inhibitors address the edema released by this pathological vasculature in most patients, with most of these becoming less responsive over time; thus, there is a need for other mechanisms to reduce the edema. More importantly, the anti-VEGF therapies do nothing to cause the regression of the pathological vessels or limit the fibrosis responsible for the long-term loss of sight. This is an unmet clinical need not addressed by any other compounds in development for AMD.
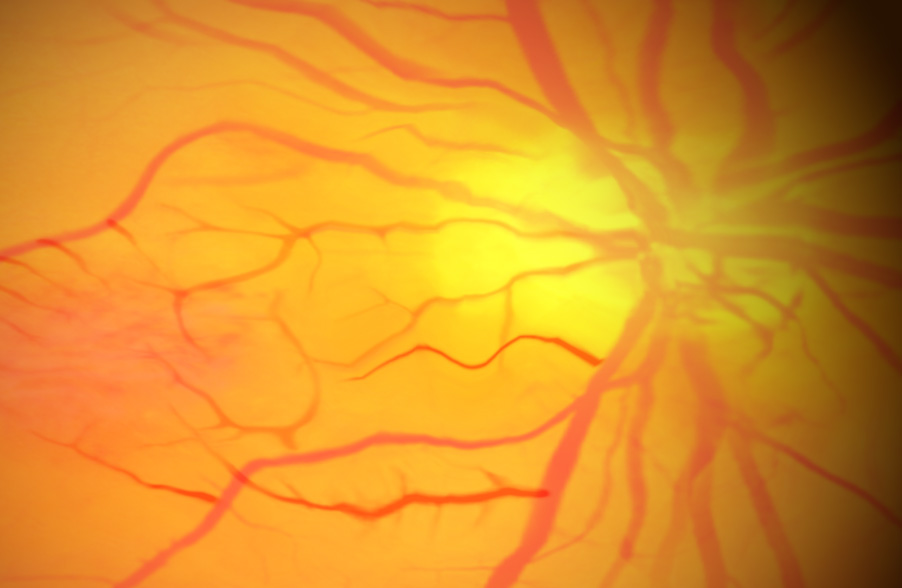
In 2021, across all ages, an estimated 9.6 million people in the United States were living with diabetic retinopathy (DR). Of these, 1.84 million were living with vision-threatening proliferative DR (pDR) or late-stage Diabetic Retinopathy. In diabetic retinopathy, abnormal, “leaky” blood vessels release fluid and can cause hemorrhages. At the advanced stage of the disease, new vessels infiltrate the retina, leading to the formation of fibrotic tissue on the retinal surface or in the vitreous cavity, vitreous hemorrhage, retinal detachment, retinal damage, and loss of vision.
These blood vessels, which are thin, weak, and prone to bleeding, cause scar tissue to form inside the eye. This scar tissue can pull the retina away from the back of your eye, causing retinal detachment. A detached retina typically results in blurriness, a reduced field of vision, and, in severe cases, permanent loss of vision. Laser photocoagulation is often effective; however, the procedure can be painful and may result in loss of peripheral vision. Anti-VEGF therapies are effective for a large percentage of patients, but as many as 25% do not respond even at the start, and many more become resistant over time.
OGX-110 has been shown to cause the regression of these nascent pathologic vessels in the animal model of pDR and to suppress the development of fibrosis. This novel mechanism of action holds great promise for improving sight in the short term and ultimately shutting down the drivers of the disease.
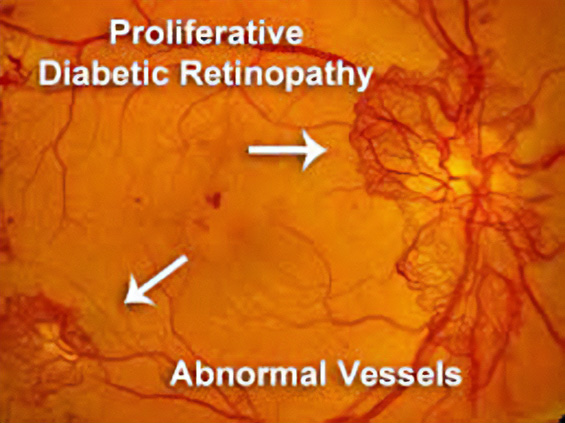
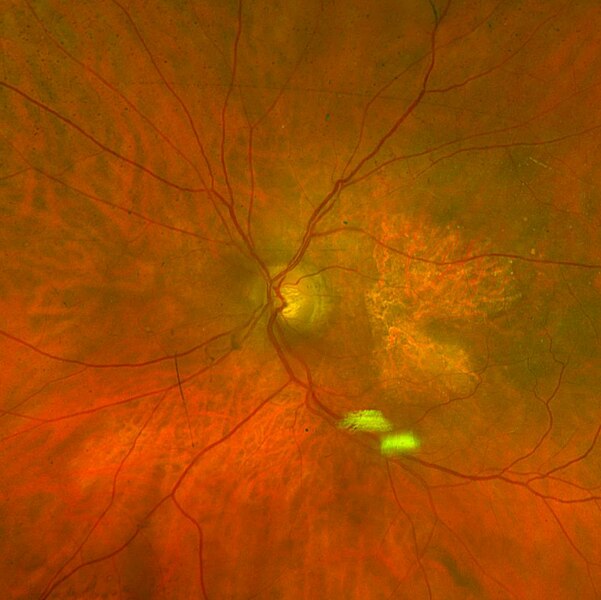
Geographic Atrophy
More than 5 million people worldwide have geographic atrophy, including nearly 1 million in the U.S. In developed nations, approximately 1 in 29 people over the age of 75 have geographic atrophy, which increases to almost 1 in 4 people over the age of 90.
With aging, the retinal pigment epithelium (RPE) is exposed to intrinsic and extrinsic oxidative and environmental stressors such as cigarette smoke. The cytotoxic damage accumulates, resulting in the formation of drusen, yellow deposits of lipids between the RPE and Bruch’s membrane. Excessive drusen accumulation and drusen components, such as cellular debris, lipids, and lipoproteins, may trigger chronic inflammation through multiple pathways, including a chronic wound response. Chronic inflammation, a result of the wound response, can eventually lead to photoreceptor, RPE, and choriocapillaris cell death, causing the appearance of sharply defined atrophic lesions that are characteristic of GA and the appearance of choroidal vessels due to the missing RPE layer.
Two therapies limiting different aspects of the complement cascade were recently approved as the first agents to treat GA. Unfortunately, each of these agents reduces the growth rate of the lesions by only about one-quarter and, therefore, does not significantly impact the retention of visual acuity, at least within the first year or two. This continued progression of the disease demonstrates a gap in treatments and argues for new mechanisms by which to shut down the chronic wound response that leads to GA.
By activating the CXCR3 pathway, OGX-110 triggers an irreversible molecular cascade that resolves the wound response, suppressing inflammation, the development of fibrosis, and the regression of the choroidal vessels.
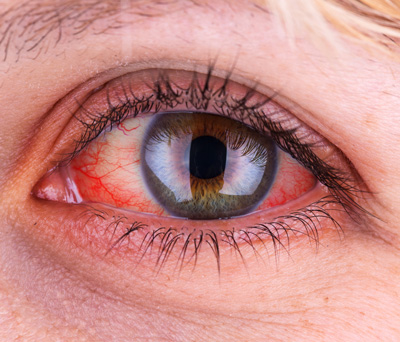
Dry Eye
Approximately 39 million patients in the United States suffer from Dry Eye Disease (DED). Symptoms include ocular pain, dryness, and a persistent, gritty sensation, which are all too familiar persistent aspects of the disease. DED can be secondary to many insults, the major ones being dry or salty air, air pollution, surgical damage of the ocular surface, and viral and autoimmune diseases. The commonality of all these causes is that a loss of tears eventually damages the goblet cells that produce the mucin that binds the tears to the eye’s surface.
There are presently five FDA-approved therapeutics for the treatment of dry eye disease. The first one, Restasis, was approved twenty years ago, but the pace of approvals has picked up significantly in the last three years. While most approved therapies focus on limiting the autoimmune activation that can lead to DED, the vast majority of DED is caused by environmental, chemical, and surgical insults. This singular focus on immune-driven DED is one of the main reasons that existing drugs have such marginal benefits.
Significant unmet clinical needs remain. The largest of these are treatments that can impact the goblet cells, the origin of mucin, and meibomian gland dysfunction (MGD), the source of the lipid layer in tears. Our animal studies demonstrate that our therapy, which causes the regeneration of goblet cells, can positively affect environmental, surgical, and chemical-based DED. As the goblet cell functioning is central to DED, and we have found that our agent can restore this function, Ocugenix is uniquely positioned to be disease-modifying in DED.
Mechanism of Action
OGX-110 selectively activates the CXCR3 pathway via the physiologic binding site. The CXCR3 pathway has been well-characterized in wound healing, regulating both fibrosis and angiogenesis. This pathway restores wound healing toward a regenerative state by noncompetitively overriding pro-angiogenic and pro-fibrotic signals from various growth factors, including VEGF.
In retinal diseases, activating this same pathway is expected to produce an anti-fibrotic and angio-regressive effect that would not only arrest disease progression but potentially reverse it. Ocu-110 is derived from a naturally occurring activator of the CXCR3 pathway, which has been vetted in preclinical and clinical studies, demonstrating its ability to regress vascularization and inhibit fibrosis. As it is a physiologic activator of CXCR3, there has been no evidence of toxicity in any of our preclinical studies.
Advantages of a New Mechanism of Action
Current therapies for the target indications are either incomplete or suboptimal. In most retinal situations, treatments consist of a single mechanism of action, unlike treatments in other indications. Treating patients with therapies that have a variety of mechanisms of action has led to significant advances in nearly all diseases, including oncology and immunology.
Patients with angio-fibrotic retinal diseases such as wet AMD and proliferative diabetic retinopathy, which are driven by a variety of growth factors, would likely benefit from alternative therapeutic approaches in addition to anti-VEGF therapies. The current anti-VEGF therapies address the edema in most patients but do nothing to halt the progression to fibrosis. Additionally, up to one-third of patients do not regain visual acuity with these therapies, and most patients will lose acuity over time, even if they initially respond. Thus, anti-fibrotic treatments and novel approaches to managing edema are needed.
In the chronic fibrotic and scarring condition of GA, the current therapies block a key driver pathway, the complement cascade. However, this only slows progression by about 25%. Other drivers and processes are clearly at work. As CXCR3 signaling overrides chronic fibrosis and restores homeostasis, Ocu-110 should act independently of the complement cascade and provide a new approach to stopping the progression of GA.
Pipeline
- Preclinical
- IND-Enabling
- Phase 1
- Phase 2
- Phase 3
Retinal Fibro-Vascular Disease
Wet AMD
- Preclinical
- IND-Enabling
- Phase 1
- Phase 2
- Phase 3
Proliferative Diabetic Retinopathy
- Preclinical
- IND-Enabling
- Phase 1
- Phase 2
- Phase 3
Geographic Atrophy
- Preclinical
- IND-Enabling
- Phase 1
- Phase 2
- Phase 3
Ocular Surface Inflammatory Disease
Dry Eye
- Preclinical
- IND-Enabling
- Phase 1
- Phase 2
- Phase 3
Company
Our Team
Sean McDonald
Position: Chief Executive Officer
Sean McDonald is a venture partner at Adams Capital Management and chief executive officer of Ocugenix. Mr. McDonald has a track record of building start-ups from early stages of venture capital investment to self-sufficient revenue generation. He previously led Precision Therapeutics, a market-leading personalized medicine company, that he grew through both internal development as well as acquisitions to $40 million in revenue. During Mr. McDonald’s tenure as chief executive officer, Precision Therapeutics developed the most diversified portfolio in the industry of validated personalized medicine products impacting lung, colon and gynecological cancers. Earlier in his career, he founded Automated Healthcare, a company that developed and marketed the first systems that automated the dispensing and administration of medications in hospital pharmacies. The system has been successfully deployed in several hundred hospital pharmacies across the country and dispenses a half a billion prescriptions annually error-free. Mr. McDonald grew that business from startup to close to $200 million in revenue. He also was one of the top five executives at McKesson Corporation for five years as well as a board member for ten years of Respironics, a medical device company purchased by Philips for $5.3 billion.
Sean McDonald
Chief Executive Officer
Sean McDonald is a venture partner at Adams Capital Management...
Alan Wells, M.D., DMSc
Position: Chief Scientific Officer
Alan Wells, M.D., DMSc, is currently the executive vice-chairman of the section of laboratory medicine (encompassing clinical chemistry, clinical microbiology, hematopathology, immunopathology, transfusion medicine, and clinical pathology throughout the UPMC Health System). He also serves as the medical director for the UPMC Clinical Laboratories. As the Thomas Gill III Professor of Pathology, Dr. Wells directs a large research endeavor investigating how cells interact with and respond to their microenvironment during cancer dissemination and wound healing, with an eye towards biologically engineered and stem cell therapeutics in these arenas.
Alan Wells, M.D., DMSc
Chief Scientific Officer
Alan Wells, M.D., DMSc, is currently the executive vice-chairman of...
Ian P. Conner, MD, Ph.D.
Position: Advisor
Ian P. Conner, M.D., PhD is an assistant professor of ophthalmology and bioengineering at the University of Pittsburgh and the director of the UPMC Eye Center. Through an unrestricted grant from the Research to Prevent Blindness (RPB), Dr. Conner has collaborated with faculty members affiliated with the McGowan Institute for Regenerative Medicine to investigate the structural, metabolic, and functional relationships between the eye and the brain in glaucoma. In addition to his research efforts, Dr. Conner has served as site investigator on several UPMC Eye Center clinical studies on glaucoma and ocular hypertension.
Ian P. Conner, MD, Ph.D.
Advisor
Ian P. Conner, M.D., PhD is an assistant professor of...
Joel S. Schuman, MD
Position: Advisor
Joel S. Schuman, M.D. is currently the Chair of Ophthalmology at NYU. Previously he was the director of the UPMC Eye Center, one of the nation’s top authorities on glaucoma and diagnostic testing for eye disease, and the Eye and Ear Foundation Professor and Chairman of Ophthalmology at the University of Pittsburgh School of Medicine. He also held secondary appointments at the McGowan Institute for Regenerative Medicine at UPMC, the Center for the Neural Basis of Cognition jointly run by the University of Pittsburgh and Carnegie Mellon University, and as professor of bioengineering at the University of Pittsburgh’s Swanson School of Engineering. Dr. Schuman helped to develop optical coherence tomography (OCT), now known as Spectral OCT, which is the most powerful tool available for early detection of glaucoma. Dr. Schuman and his colleagues were the first to identify a molecular marker for human glaucoma, as published in Nature Medicine. This discovery has paved the way for other significant advances in the treatment and diagnosis of glaucoma.
Joel S. Schuman, MD
Advisor
Joel S. Schuman, M.D. is currently the Chair of Ophthalmology...
Cecelia C. Yates, Ph.D.
Position: Scientific Advisor
Cecelia Yates, PhD, is an associate professor of health promotion and development at UPMC School of Nursing. Dr. Yates’ research focuses on chemokine and extracellular matrix interactions in systemic sclerosis (scleroderma) and idiopathic pulmonary fibrosis in the development of dermal and pulmonary fibrosis. She has a cellular and molecular laboratory fully equipped for basic and translational research located within the School of Nursing. Current members of the laboratory are investigating genomic regulation of molecules that mediate the development and progression of fibrosis. Dr. Yates is also developing small molecule therapeutics and cellular therapies to improve tissue remodeling.
Cecelia C. Yates, Ph.D.
Scientific Advisor
Cecelia Yates, PhD, is an associate professor of health promotion...
Clinical Advisors

SriniVas Sadda, MD, FARVO
- President, Association for Research in Vision and Ophthalmology (ARVO)
- Director, Artificial Intelligence and Imaging Research at the Doheny Eye Institute
- Professor of Ophthalmology, UCLA

David Boyer, MD
- Senior Partner, Retina-Vitreous Associates Medical Group
- Adjunct Clinical Professor of Ophthalmology, Keck School of Medicine of USC

Roger Goldberg, MD, MBA
- Vitreoretinal Surgeon, Bay Area Retina Associates
- Active Member, American Society of Retinal Specialists, the Retina Society, and the American Academy of Ophthalmology

Sunil Patel, MD, PhD
- Vitreoretinal Surgeon and Managing Partner, Ophthalmology Specialist of Texas
- President, Integrated Clinical Research

Raj Maturi, MD, FASRS
- Founder and Partner, Retina Partners Midwest
- Adjunct Clinical Associate Professor of Ophthalmology, Indiana University School of Medicine

Robert Avery, MD
- Founder and CEO, California Retina Consultants
- Co-Founder, California Retina Research Foundation

Sunil Srivastava, MD
- Uveitis and Vireo-Retinal Specialist, Cole Eye Institute at the Cleveland Clinic
- Senior Achievement Award Recipient, American Academy of Ophthalmology
Contact Us
OCUGENIX
Pittsburgh, PA 15232
Sean McDonald
President & CEO
sean@ocugenixtx.com
CALL US









